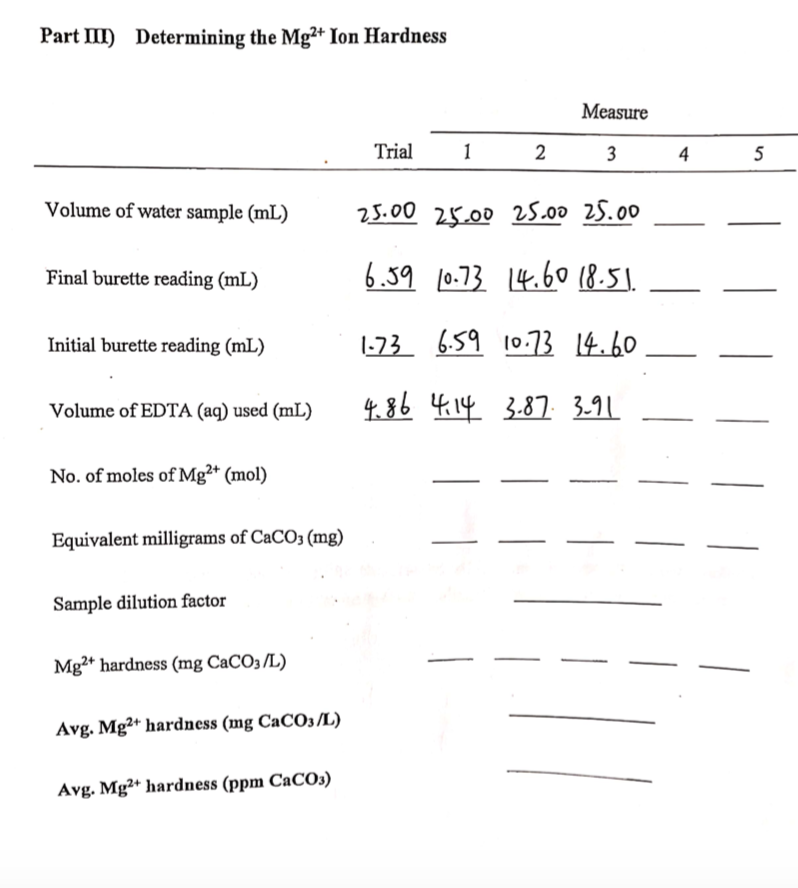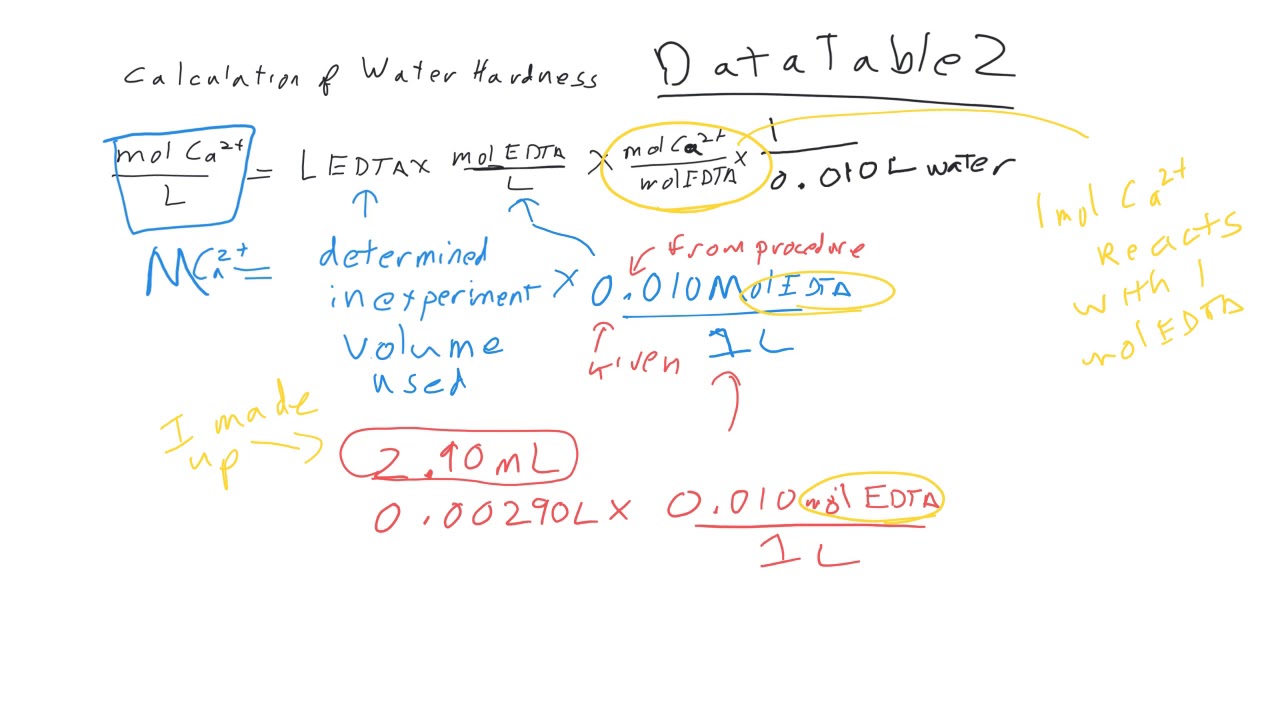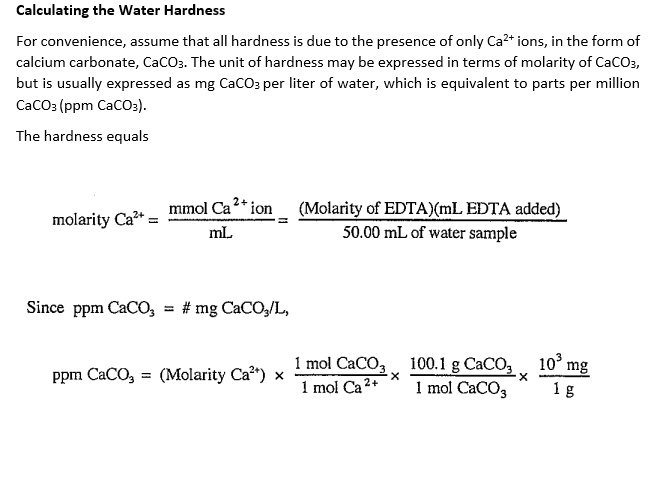
SOLVED: Calculating the Water Hardness For convenience assume that all hardness is due to the presence of only Caz+ions in the form of calcium carbonate, CaCO3 The unit of hardness may be

SOLVED: on 1i (These are post-lab calculations from the Water HardnessLab) Calculate the concentration of calcium in the calcium standard (inunitsppm Ca)used for the Water Hardness Lab and calculate the ppm calcium
![SOLVED: 6. Calculate the total hardness, calcium hardness and magnesium hardness for a water sample having a pH of 7.2 and the following concentrations of ions: [Ca ] = 40 mgL [Mg ] = SOLVED: 6. Calculate the total hardness, calcium hardness and magnesium hardness for a water sample having a pH of 7.2 and the following concentrations of ions: [Ca ] = 40 mgL [Mg ] =](https://cdn.numerade.com/ask_previews/32bca3e4-ee47-44d7-8fb8-7e86804add2d_large.jpg)
SOLVED: 6. Calculate the total hardness, calcium hardness and magnesium hardness for a water sample having a pH of 7.2 and the following concentrations of ions: [Ca ] = 40 mgL [Mg ] =

21 Water Softening Calculating Calcium H - 211 21 Water Softening TOPICS Water Hardness Calculating - Studocu

SOLVED: Problems Calculate total hardness, carbonate hardness, and non-carbonate hardness of the samples What is the degree ofhardness of the sample (sof, moderate; hard, very hard)? Why might we necd to know
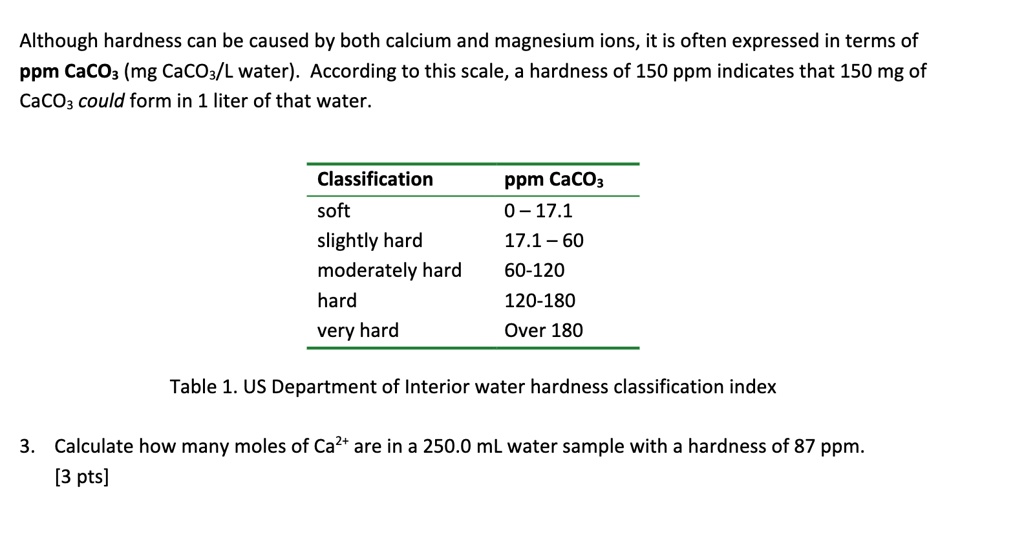
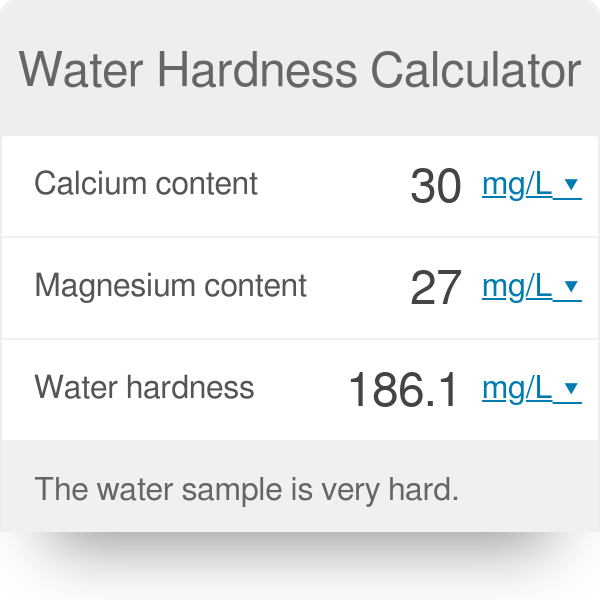
SOLVED: Although hardness can be caused by both calcium and magnesium ions, it is often expressed in terms of ppm CaCOz (mg CaCOz/L water) According to this scale, a hardness of 150

One litre of sample of hard water contain 4.44mg CaCI2 and 1.9mg of MgCI2. What is the total hardness in terms of ppm of CaCO3 ?

Hardness Objective n to understand the chemical basis of water hardness, how it originates, and ways it can affect water distribution systems. n to know. - ppt download
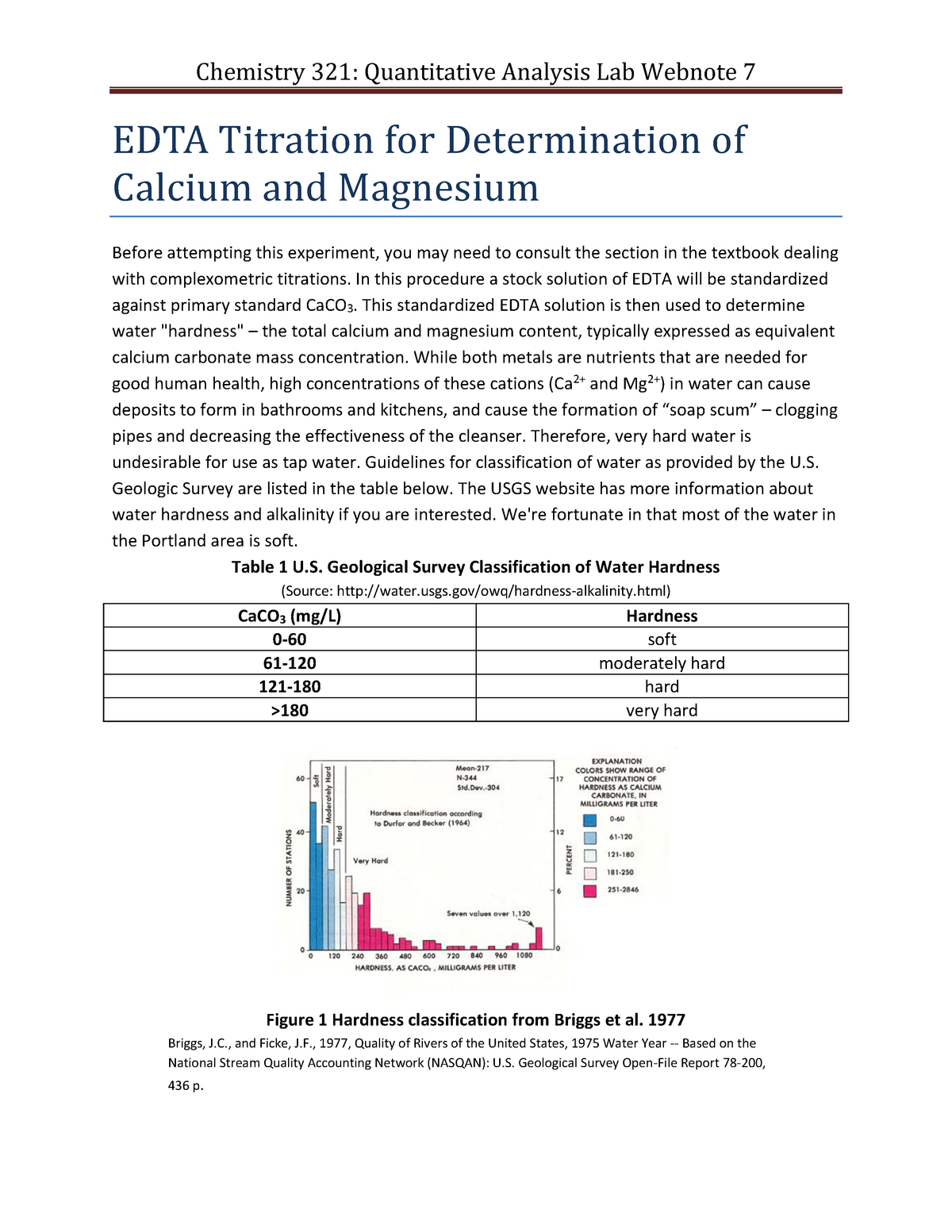
EDTA Titration for Determination of calcium and magnesium - In this procedure a stock solution of - Studocu

Hardness CE Lab. Definition Hardness of water is a measure of its capacity to precipitate soap and is caused mainly by the presence of divalent. - ppt download

Naturally Occurring Elements in Nebraska's Groundwater: Part 1 of a Series - Calcium and Magnesium | UNL Water